Ultra-thin Heteroprotein Films
September 21, 2023 2023-09-21 13:42Ultra-thin Heteroprotein Films
Ultra-thin Heteroprotein Films
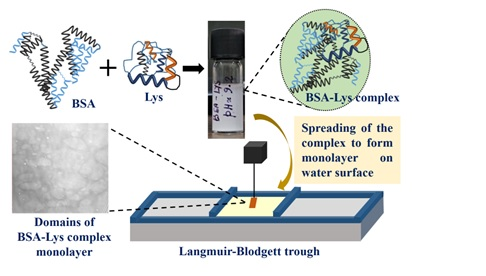
Recently, Scientists have successfully developed ultrathin monolayer protein films consisting of two globular proteins: bovine serum albumin (BSA) and lysozyme (Lys). They used the Langmuir-Blodgett (LB) technique which gives the film thickness in the order of nanometers.
✅Scientists from the Institute of Advanced Study in Science and Technology (IASST) have developed ultra-thin monolayer heteroprotein films.
✅Formation – These ultra-thin heteroprotein films were usually developed from bulk solutions.
In the above-mentioned study, the ultrathin monolayer protein films were developed consisting of two globular proteins:
🔸Bovine serum albumin (BSA) and
🔸Lysozyme (Lys).
✅This is the first time the Langmuir-Blodgett (LB) technique was used to produce these films, which gives the films the thickness in nanometers.
✅As a result of electrostatic attraction & hydrophobic interactions between the two proteins, a complex was formed at a pH of 9.2.
✅This monolayer complex was formed at the air-water interface, which was later transferred to the silicon substrates.
✅Advantages – These soft heteroprotein films of the BSA and Lys are more flexible than other protein or plastic films.
These films can be used to fabricate highly stable biodegradable thin films of different protein complexes for expanding its applications in the area of thin-film technology.
✅The monolayers at the air-water interface can hold their intrinsic structure for a sufficiently longer time period due to the complexation forming a highly stable film.
These films have excellent thermal, mechanical and pH stability.
✅So, they can pave the way for expanding applications of thin films in biomedical and food packaging industries.
✅To make the protein film free standing for diverse applications, diverse physicochemical methods such as
Parameter alteration or
Incorporation of different fatty acids or polyol moieties (glycerol, starch, gelatin, etc.) into this complex can be done.